Red dye made from bugs moves to lab
After increasing demand for the popular red dye, Cochineal, biochemists are currently recreating the dye, normally made from bugs, in the lab.
Wikimedia under creative commons license
Someone harvesting and grinding cochineal bugs from cacti into the carmine or cochineal dye used in many products from makeup products to food.
After increasing demand for the popular red dye, Cochineal, biochemists are currently recreating the dye, normally made from bugs in the lab.
What is Cochineal Dye?
At Britannica.com, there is a brief explanation of cochineal or carmine dye. It is a naturally made dye from the oval-shaped cochineal bugs that are native to tropical and subtropical America. This dye produces red-tinted dyes such as crimson and orange. While mostly used in cosmetics and beverages, cochineal dyes are also used in dyes for textiles, drugs, and food. From lipstick to pink pastries.
The traditional way of harvesting these bugs to produce the dye involves brushing them off of cacti into a bag and then immersing them in hot water, steam, or a hot oven. The ability of cochineal dye to dye things is caused by this boiling water. Mills that are the size of phone booths are used to grind the bugs to powder and then the powder is paired with salts to isolate the carmine to make the dye. An estimated 70,000 cochineal bugs are needed to make one pound of dried insect, and a fifth of a pound of carmine acid to make the dye itself with the traditional methods that many have been using. According to Knowable Magazine and Smithsonian Magazine, Peru is the largest commercial producer of the dye, followed by Mexico, where Biotechnologist Portillo Martinez from the University of Guadalajara talked about making the dye: “It is time-consuming labor, but there is something funny about it: If you start working with cochineal, you fall in love with it.”.
However, now due to high demand, there is a strain between how much is being needed to meet that demand and the traditional harvesting methods that have been being used. In response, Biotechnologists are looking for a way to bring the dye to the laboratory to make a synthetic version to better meet demand.

But why has the demand for the dye been rising?
The demand for this red dye has been increasing in recent years due to people’s wishes to have more “natural” flavorings or dyes as opposed to those that are not considered “natural,” such as those that are petroleum-based. Many people hold the Carmine dye as a renewable natural resource that is much safer. According to some sites like Axiology, demand has also increased for an alternative product of the dye to create vegan, cruelty-free versions of products that people consume. Demand is not always strictly on the rise, the demand of the dye has taken a decline as well. As some studies as documented by the Smithsonian Magazine have reported allergic reactions and health concerns related to cochineal dye. However, overall global demand for the dye is suspected to rise for companies looking to use it to color their products, mostly food, from drinks to meats. Causing the price of cochineal to rise as much as 40 percent in some places per ton. Therefore, biochemists are now looking for a way to make the cochineal dye in the lab.

One biomedical dye chemist, D. Dapson, states in Smithsonian Magazine that a reason for this change is that, “Habitat for cacti is limited, growth of both host and parasite are slow, and extraction procedures are woefully inefficient.”
He also continues by stating, “Improvements in extraction and purification have been made, but they don’t address the core problem, which is the production of the insects.” In addition to making it in the lab, there are more possibilities to be had, with being able to create better colors and better biological activity. However, just like the issues with the traditional way of harvesting, there are also challenges with creating a version in the lab.
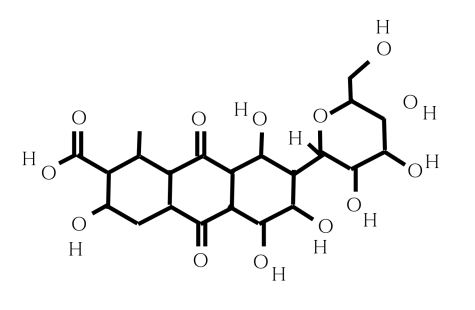
According to Smithsonian Magazine, the first challenge is that the acid that makes the dye has a complicated structure called anthraquinone. A central ring structure with glucose and other chemical groups makes it difficult to synthesize in large amounts. Biochemists and scientists still don’t fully understand the full biochemical pathway cochineal insects use to make the compound. But despite these challenges, scientists and biochemists are still determined to make an alternative. The process began ten years ago and, through trial and error, biochemists have created a three-ring core of carminic acid from fungus, as well as another way of taking a gene from the insect itself and using it to convert Kermesic to carminic acid.
Chemist Frandsen stated, “It was a long struggle with lots of things that should work in theory, but which did not in the real world. The truth about synthetic biology is that it is very early days, and results are often presented as ‘we just did it and it was easy’, while the reality is way different.”
While there is still a lot of work to be researched and done before this process of making synthetic cochineal can be increased to industrial levels, Frandsen and other biochemists remark that some companies are already interested. This new way of making this dye could quell some concerns people have over products made from animals, as well as reduce fears people have of genetically modified foods. At the end of the day, scientists, biochemists, and companies alike seem to be hopeful about this new version of the dye.

Hello, my name is Maggie Brands. I'm a senior. I'm new here so I'm excited to see what will happen in this class and what we do here. I enjoy reading and...